Why Does Increasing Surface Area Increase the Rate of Dissolving
Stirring distributes the solute throughout the solution and therefore increases the rate of dissolution. Because the solvent can get to more of the solute quicker higher surface area INCREASES the rate of dissolution.

Rate Of Dissolving Increase The Rate Surface Area Stir Temperature Straight Science Youtube
We would do this to increase how quickly the solute would dissolve in solution.

. The only place that dissolution can take place is at the surface of a solid. In this video we look at how to increase the RATE a solute dissolves into a solvent. The rate of dissolution simply tells us how fast or slow a given solute will dissolve in a particular solvent.
How does surface area affect the rate of dissolving. Increasing the surface area of a solid C increases the speed of dissolving. We would do this to increase how quickly the solute would dissolve in solution.
Conclusions If we were to increase the surface area of a solid then it would have been broken into smaller pieces. Decreases rate of dissolution of a solid. Increased Surface Area Increases Rate of Dissolving Another factor that affects the rate of solubility is the surface area of a solid.
How fast or slow a solid dissolves in a solvent the amount of time it takes agitate. Iii Particle Size Crushing solute into smaller pieces increases the surface area that is in contact with solvent thus increasing the rate of dissolving. So the bigger amount of surface area exposed to the celebrant the quicker it dissolves.
Please make sure you understand that the only thing we are affecti. When expanding alveolis surface area is increased and the diffusion rate is faster. Answer 1 of 3.
Dissolving is a surface phenomenon since it depends on solvent molecules colliding with the outer surface of the solute. Expanding the surface area builds the pace of dissolvability of a strong on the grounds that a bigger number of atoms of the more prominent surface region have contact with the dissolvable. The greater the surface area of the solute the faster the rate of dissolution of the solute.
A given quantity of solute dissolves faster when it is ground into small particles than if it is in the form of a large chunk because more surface area is exposed. This is because there is more area for the solvent to contact the solute and thus the solvent is able to interact with more of the solute at one time and dissolve it quicker. Greater Surface Area.
Thereof why do alveoli have a large surface area. Up to 24 cash back increasing collisions and the rate of dissolving. As the surface area of a solute increases so does its solubility.
The textbook uses a. If we were to increase the surface area of a solid then it would have been broken into smaller pieces. The Dissolving Process Whether or not a solute dissolves and to what extent depends on the forces of attraction between.
The packet of granulated sugar exposes far more surface area to the solvent and dissolves more. But they put it on a small table in the. You are at a party and they have a full sheet cake cut into 100 pieces for 100 guests.
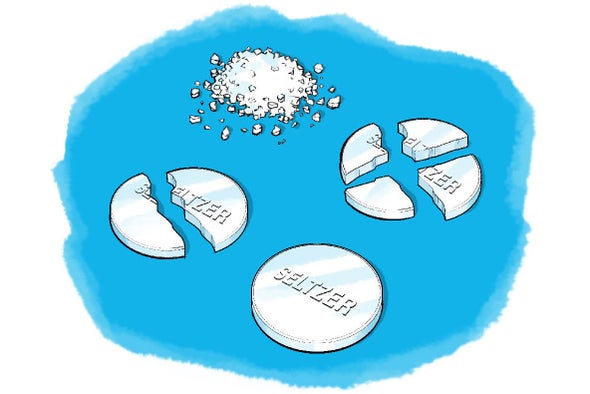
An increase in the surface area affects the Alka- Seltzer tablets by increasing the rate at which it dissolves. The more surface area or exposed area results in more sites that contact water immediately resulting in the antacid dissolving faster and the chemical reaction fizzing happening faster. More particles are exposed to the other reactant there is a greater chance of particles colliding which leads to more successful collisions per.
Okay this is question nine from chapter 15 Section one. Therefore according to our results it is not one of the factors that make an Alka- Seltzer tablet dissolve faster without physically stirring it. Research and list all the factors that affect the rate at which a solid dissolves in a liquid.
Explain why or why not. To shake or stir a solution. Increases rate of dissolution of a solid.
The crushed tablet has the smallest pieces and thus the highest surface area to volume ratio causing it to react the fastest. There are four main factors that affect this rate. Crushing increases the amount of surface area exposed to the solvent.
Temperature stirring area of. The layer of moisture in the alveoli allows gases to dissolve so that they can diffuse quickly. And this question is asking why does an increase in surface area close an increasing rate of dissolving.
If the surface area of a reactant is increased. Greater surface areas of solids help them dissolve faster. Think about a cube of sugar and a sheet of sugar each the same mass.
Why does increasing the surface area of a solid solute help it to dissolve more quickly. Increasing the surface area of a solid solute speeds up the rate at which it dissolves in a liquid solvent Increased SA exposes more solute to solvent allows solvent to come in contact with more solute in less time.
React Fast How Size Determines Rate Scientific American

Dissolving Science Educational School Posters School Posters Chemistry Classroom Education
No comments for "Why Does Increasing Surface Area Increase the Rate of Dissolving"
Post a Comment